Meningitis Vaccine
A huge breakthrough in the fight against the most serious form of meningitis
What’s the big deal?
The charity Meningitis UK, estimates that there are 1,870 cases of meningitis each year in the UK. Meningitis is most common in children under five years old, and in particular in babies under the age of one. This disease kills around 1 in 10 of those affected and a significant proportion of survivors are left with life-affecting problems.
What is this vaccine for?
The vaccine gives protection against the most common type of bacterial meningitis and septicaemia in the UK. There are other types of meningitis that will not be prevented by this vaccine. It is vital that everyone knows the signs and symptoms of meningitis and septicaemia, and gets urgent medical help if they are concerned.
Why do we need a vaccine, can’t we protect against meningitis in any other way?
Bacterial meningitis and is a life-threatening disease. The only effective way to prevent these diseases is through vaccination.
Is the vaccine safe?
It was licensed by the European Medical Association in January 2013 and all vaccines are extensively tested for safety and effectiveness before being licensed.
How many children were in the trials?
Approximately 7,500 infants, children, adolescents and adults.
How effective would this vaccine be and does it cover all strains?
This vaccine is the first one to demonstrate potential coverage of (protection against) the majority of strains. However, the vaccine will not protect against all cases, but coverage estimates show that 73% of infection will be covered.
Why doesn’t it cover all strains?
There are many different strains. This vaccine has been developed to offer protection against as many as possible. Once it has been in use for some time, it will be possible to calculate the coverage and continue with vaccine research to improve the protection it gives. Knowing the signs and symptoms and seeking early medical help remain vital in saving lives. Clinical trials have shown that this vaccine (as do all vaccines) stimulates the body’s immune system to generate protective antibodies.
Does the vaccine have any known side-effects?
In infants and children (less than 2 years of age) the most common adverse reactions observed in clinical trials were tenderness and swelling at the injection site, fever and irritability. Taking over the counter painkillers at the time of vaccination or shortly afterwards can help reduce the risk of such reactions. Remember painkillers should only be taken as instructed.
Can the vaccine actually cause meningitis? Is it live?
No, the vaccine cannot cause meningitis. It is inactivated.
How effective are the meningitis vaccines that are currently in use?
Over the past 20 years, vaccines that prevent different types of meningitis have been introduced into the childhood immunisation schedule and also given to others at increased risk. These vaccines have prevented thousands of cases; saving lives and significantly reducing the number of people living with life-long disabilities.

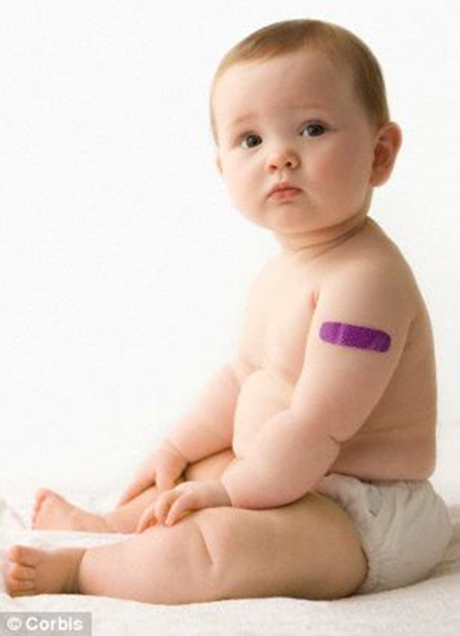
Could this vaccine be given to new-born babies born to mothers with GBS?
The vaccine will not protect new-born babies from GBS (Group B Streptococcal infection). Work is being undertaken on a vaccine for GBS, but it is likely to be many years before a vaccine for GBS is readily available.
My son had meningitis type B. Can he have the vaccine as I’m aware he could have meningitis again?
Most people who have suffered from bacterial meningitis will have developed some immunity against the type of bacteria that caused their illness. However, as there are different causes of meningitis, it is possible though rare, to contract the disease more than once.
Were any unusual side effects seen in the trials?
Like all vaccines, this vaccine can cause side effects although not everybody gets them.
Uncommon side effects, described as up to 1 in 100 people, are high fever, seizures (including febrile seizures), vomiting (after booster) dry skin, itchy rash, skin rash, paleness (rare after booster).
Rare side effects (may affect up to 1 in 1,000 people), Kawasaki syndrome (a rare condition that mainly affects children under the age of five. It is also known as mucocutaneous lymph node syndrome).
Does the vaccine it contain latex?
A dose of vaccine is presented as follows:
A 0.5 ml suspension in a pre-filled syringe (Type I glass) with a plunger stopper (Type I bromobutyl rubber) and with a protective tip cap (Type I or Type II rubber with or without needles).
